3,5-Dimethylpiperidine is very important in modern organic synthesis. Chemists use it as a building block, intermediate, and catalyst. Many medicines and special materials need its nitrogen-containing structure. Recent studies show its molecular structure and electronic properties using advanced tools. Its derivatives have many uses in drugs and materials, which makes it valuable.
Key Takeaways
- 3,5-Dimethylpiperidine is a useful compound. People use it to help make medicines, materials, and chemicals. It can be a building block, an intermediate, or a catalyst.
- It has two isomers called cis and trans. These have different shapes. The shape changes how it works in reactions. Chemists pick the right one for each job.
- Today, people make 3,5-Dimethylpiperidine in safe and smart ways. They use green chemistry and special machines called continuous reactors. This saves energy and makes less waste.
- This compound helps drugs work better and last longer. It also makes materials stronger and reactions faster. That is why it is important for medicines, plastics, and special materials like zeolites.
- You must be careful when using 3,5-Dimethylpiperidine. But it is usually safe in labs if you store it right. Suppliers can send different amounts quickly for many uses.
3,5-Dimethylpiperidine Overview
Structure and Isomers
3,5-Dimethylpiperidine is part of the piperidine family. It has a ring made of six atoms. There are two methyl groups at spots 3 and 5 on the ring. This setup makes two main isomers called cis and trans. These isomers are different because of where the methyl groups are. In the cis isomer, both methyl groups are on the same side. In the trans isomer, they are on opposite sides.
Chemists have looked at these isomers in many ways. They use hydrogenation reactions with special catalysts to make both cis and trans forms. For example, using 10% palladium on carbon gives more trans than cis. The ratio is 70 parts trans to 30 parts cis. If they use 10% platinum oxide, the ratio changes to 60 parts trans and 40 parts cis. Epimerization reactions can change these ratios even more. Potassium tert-butoxide in tetrahydrofuran at cold temperatures can help make more of one isomer. It depends on which isomer you start with. Scientists check the structures with NMR and single-crystal X-ray diffraction.
| Experiment Type | Conditions / Catalyst | Isomer Ratio (dr) | Isolated Yields (%) | Structural Confirmation Method |
|---|---|---|---|---|
| Hydrogenation | 10% Pd/C | trans:cis = 70:30 | trans-5j: 51%, cis-5j: 17% | N-benzylation followed by isolation |
| Hydrogenation | 10% PtO₂ | trans:cis = 60:40 | N/A | N/A |
| Epimerisation (cis to trans) | KOtBu in THF, -78 °C, 2 h | 50:50 to 95:5 (trans favored) | 40–90% | NMR J values, comparison with known compounds |
| Epimerisation (trans to cis) | KOtBu in THF, -78 °C, 2 h | 85:15 (cis favored) | 62% | N/A |
| Stereochemical confirmation | Single-crystal X-ray diffraction (N-tosyl derivative of cis-5h) | N/A | N/A | X-ray diffraction |
Note: Chemists can change the isomer ratios. This helps them use 3,5-dimethylpiperidine for special jobs.
Key Properties
3,5-Dimethylpiperidine is stable and useful. It can be a colorless liquid or a solid. This depends on the temperature. It mixes well with many organic liquids. Both isomers react in similar ways, but their physical traits can be different. For example, melting and boiling points can change with the isomer.
This compound does not get oxidized easily. Its nitrogen atom acts as a base in many reactions. The methyl groups at spots 3 and 5 make the molecule bigger. This can change how it reacts with other chemicals. Chemists like this when they want certain reactions to happen.
3,5-Dimethylpiperidine is not very toxic in most labs. If you handle and store it right, it is safe to use. Its special mix of traits makes it a top pick for making complex molecules in modern organic synthesis.
Functions in Synthesis
Building Block
3,5-Dimethylpiperidine is a main building block in many syntheses. Chemists use it to make medicines, APIs, and special chemicals. Its nitrogen ring and methyl groups help build complex molecules. These parts let chemists make molecules with great accuracy. Drug makers use it to make CNS drugs work better. These drugs can be up to 3.5 times more selective than others. In fungicides, this compound helps products last longer in fields. Farmers do not need to spray as often because it lasts 40% longer.
The table below shows some ways 3,5-Dimethylpiperidine helps as a building block:
| Application Area | Documented Improvement | Metric / Yield Enhancement |
|---|---|---|
| CNS Drug Development | Increased target selectivity | 3.5 times higher selectivity |
| Fungicide Development | Longer persistence, reduced application | 40% longer persistence |
| Asymmetric Hydrogenation Catalyst | Higher enantiomeric excess | 98.5% ee (vs. <85% typical) |
| Lubricant Additive | Improved thermal stability | 30% improvement |
| Coating Development | Enhanced corrosion resistance | 65% improvement in salt-spray testing |
Note: The cis and trans isomers can change these results. Chemists pick the right isomer for the job.
Intermediate Roles
Chemists use 3,5-Dimethylpiperidine as a reactive intermediate. It helps make additives, polymers, and new materials. Its structure lets it react fast with other chemicals. The methyl groups at spots 3 and 5 guide the reaction. This helps make the product they want. In making special additives, it gives higher yields and better stability.
For example, in lubricant additives, it boosts thermal stability by 30%. This means the product can handle more heat before breaking down. In coatings, it helps make materials that fight rust. Salt-spray tests show a 65% better resistance when this compound is used.
Chemists like to control which isomer they use. The cis isomer can give different results than the trans isomer. This control helps make products for special uses.
Catalyst Uses
3,5-Dimethylpiperidine works as a catalyst in many reactions. Its nitrogen atom gives electrons to speed up changes. In asymmetric hydrogenation, it helps make molecules with high enantiomeric excess. Some reactions reach 98.5% enantiomeric excess, which is much higher than usual.
Researchers often use the trans isomer for these jobs. The shape of the molecule can change how well it works. Picking the right isomer makes the reaction faster and more exact.
Chemists keep finding new ways to use 3,5-Dimethylpiperidine as a catalyst. Its special structure and isomers make it a great tool in organic synthesis.
Applications
Pharmaceuticals
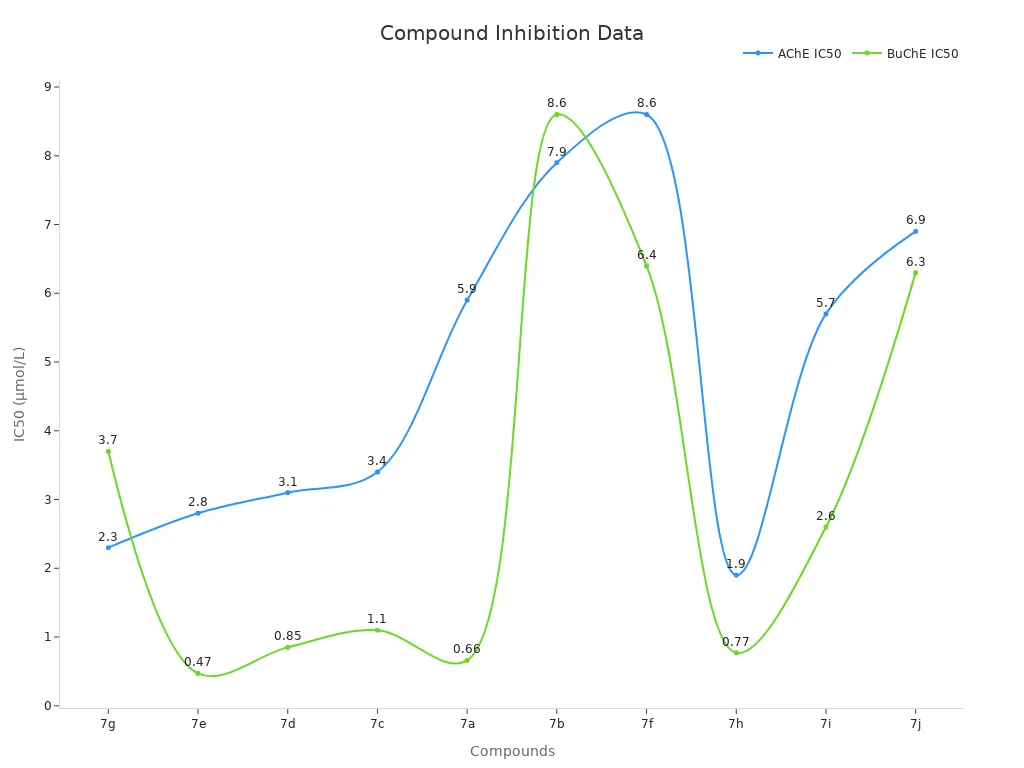
3,5-Dimethylpiperidine is important in making medicines. Chemists use it to help build new drugs and APIs. Its shape helps make compounds that can target enzymes. Some scientists test 3,5-Dimethylpiperidine derivatives to block enzymes like acetylcholinesterase and butyrylcholinesterase. These enzymes are linked to diseases like Alzheimer’s.
The table below shows how well different compounds with 3,5-Dimethylpiperidine work in enzyme tests:
| Compound | Amino Fragment / NR1R2 Fragment | IC50 against Electrophorus electricus AChE (μmol/L) | IC50 against equine serum BuChE (μmol/L) | Notes |
|---|---|---|---|---|
| 7g | 3,5-Dimethylpiperidine | 2.3 | 3.7 | Moderate inhibitory potency |
| 7e | N-ethylbenzylamine | 2.8 | 0.47 | Most potent BuChE inhibitor among tested compounds |
| 7d | 1,2,3,4-Tetrahydroisoquinoline | 3.1 | 0.85 | Strong BuChE inhibition |
| 7c | 4-(3-Phenylpropyl)piperidine | 3.4 | 1.1 | Moderate BuChE inhibition |
| 7a | 4-Benzylpiperidine | 5.9 | 0.66 | Strong BuChE inhibition |
| 7b | Benzylpiperazine | 7.9 | 8.6 | Moderate BuChE inhibition |
| 7f | Diethylamine | 8.6 | 6.4 | Moderate BuChE inhibition |
| 7h | Methoxy-substituted 7e derivative | 1.9 | 0.77 | Slightly decreased BuChE activity compared to 7e |
| 7i | 4-Amino-1-benzylpiperidine (from 7h) | 5.7 | 2.6 | Decreased BuChE activity |
| 7j | 1-(4-Fluorophenyl)piperazine (from 7h) | 6.9 | 6.3 | Decreased BuChE activity |
Compounds with 3,5-Dimethylpiperidine can block enzymes at low amounts. This makes them good for more drug studies, especially for brain diseases.
Chemists also use 3,5-Dimethylpiperidine to start making drugs like tibric acid. Tibric acid helps lower cholesterol. The compound can form different isomers. This lets chemists change how a drug works or how long it lasts. In farming, its derivatives help make safer and better crop protection products.
Polymers and Additives
3,5-Dimethylpiperidine is useful for making special plastics and additives. Its nitrogen ring and methyl groups help materials last longer and work better. Factories use it to make additives for lubricants and coatings. These additives help things last longer when hot or under stress.
- Lubricant additives with 3,5-Dimethylpiperidine can handle 30% more heat.
- Coatings with its derivatives stop rust better, with a 65% improvement in tests.
Chemists also use this compound to make plastics with special features. The cis and trans isomers let them control how strong or bendy the plastic is. This helps make new plastics for electronics, cars, and planes.
Using 3,5-Dimethylpiperidine in additives and plastics helps products meet tough rules for safety and strength.
Zeolites and Materials
3,5-Dimethylpiperidine is important for making advanced materials like zeolites. Zeolites are tiny minerals with holes that help in cleaning and chemical reactions. Chemists use 3,5-Dimethylpiperidine derivatives to help shape zeolites.
To make pure 3,5-Dimethylpiperidine derivatives, chemists add it slowly to sodium hydroxide and 1,4-dibromobutane. They heat the mix, then clean it. The final product is ready for making zeolites. This careful process makes sure the material is good enough for factories.
The table below lists main features of zeolites made with 3,5-Dimethylpiperidine:
| Parameter | Description / Result |
|---|---|
| Synthesis method | Solvent-free synthesis of aluminosilicate SSZ-39 zeolite using N,N-dimethyl-3,5-dimethylpiperidine (DMDMP) as OSDA |
| Product yield | High product yield reported, with enhanced utilization efficiency of raw materials and space |
| Crystallinity | High crystallinity confirmed by powder X-ray diffraction (XRD) |
| Morphology | Uniform sheet-like morphology observed via scanning electron microscopy (SEM) |
| Surface area | Large surface area demonstrated by N2 sorption analysis |
| Acidity | Strong acidic sites identified by NH3-temperature-programmed desorption (NH3-TPD) |
| Hydrothermal stability | Excellent hydrothermal stability confirmed |
| Catalytic performance | Cu-exchanged SSZ-39 zeolite shows good catalytic activity in NH3-SCR reaction, comparable to hydrothermal synthesis |
| Environmental advantage | Solvent-free route reduces pollutants and simplifies synthesis |
Zeolites made with 3,5-Dimethylpiperidine have strong acidity and are very pure. These traits make them great for speeding up chemical reactions, like removing nitrogen oxides from air. Making zeolites without solvents is better for the environment and saves resources.
3,5-Dimethylpiperidine helps create new materials that work well and are better for the planet. This supports new ideas in chemistry and environmental science.
Synthesis Methods
3,5-Dimethylpyridine Hydrogenation
Chemists make 3,5-dimethylpiperidine by adding hydrogen to 3,5-dimethylpyridine. They use a metal catalyst like ruthenium on carbon, called Ru/C. The reaction happens in a special container with hydrogen gas. Chemists must watch the temperature and pressure closely. The type of catalyst and how they set up the reaction changes how much product they get. Using Ru/C with 3% ruthenium works very well. Chemists check the reaction with X-ray diffraction and scanning electron microscopy. These tools help them see if the catalyst is good and if the product forms right.
Isomer Separation
Hydrogenation makes both cis and trans isomers of 3,5-dimethylpiperidine. These isomers are not the same. They have different properties. Chemists separate them by crystallization or chromatography. In the cis isomer, both methyl groups are on one side. In the trans isomer, the methyl groups are on opposite sides. Picking the right isomer can change how the compound works. Careful separation helps chemists use each isomer for its best job.
Chemists can pick and separate cis or trans isomers. This lets them make molecules that do special things.
Green and Continuous Processes
Modern ways to make this compound use green chemistry and continuous methods. Continuous trickle-bed reactors, or TBRs, are now common in labs and factories. These reactors have many good points:
- They give higher conversion rates and keep the catalyst working well.
- They use lower temperatures and pressures, which saves energy.
- They are easier to use for big factory jobs.
- They help mix and heat things better, so hydrogenation works well.
- The catalyst lasts longer when used all the time.
Chemists use special tools to study catalysts, like BET surface area analysis, ICP, and TEM. By changing the size and amount of catalyst, they make reactions work better. Continuous TBRs use less solvent and finish faster than old batch methods. This way is better for the environment and fits what modern factories need.
Advantages
Efficiency
3,5-Dimethylpiperidine helps reactions work faster and better. Chemists like it because it gives high yields. Some reactions finish in just 12 hours with up to 100% yield. Other steps, like benzylation, take longer but still work well. The chart above shows how well each step does with this compound. Catalytic alkylation with ASM-40 aluminosilicates gives a 97% yield. This is much higher than older ways. It means less waste and more product each time.
Sustainability
Sustainability is important in chemistry today. 3,5-Dimethylpiperidine helps by making fewer byproducts and making catalysts last longer. Using ASM-40 aluminosilicates gives better yields and less unwanted stuff. This saves resources and helps the environment. Continuous reactors also use less energy and make less waste.
| Synthesis Method | Yield (%) | Key Advantages |
|---|---|---|
| Catalytic Alkylation (ASM-40) | 97 | High selectivity, scalable, improved catalyst longevity |
| Traditional Alkylation (ZSM-5) | 63.1 | Scalable, but more byproducts |
| Hantzsch Synthesis | 50–60 | Mild conditions, but lower atom economy |
Chemists like these changes because they make big production cleaner and cheaper.
Selectivity
Selectivity means chemists can control reactions better. 3,5-Dimethylpiperidine helps them pick where changes happen in a molecule. For example, site-selectivity ratios can be as high as 3.4:1 in C–H arylation. Some reactions give up to 95% yield with great control over the product. Researchers use NMR to check their results and make sure the right isomer forms.
| Parameter | Value / Observation |
|---|---|
| Site-selectivity ratio | 3.4:1 for C–H arylation (60% yield) |
| Diastereomeric ratios | Good ratios for regioisomers (18% yield for one regioisomer) |
| Yield and selectivity (urea from cis-3,5-dimethylpiperidine) | 95% yield, excellent trans selectivity for trisubstituted derivatives |
These results show that 3,5-dimethylpiperidine helps chemists get the products they want. This is very important for making new medicines and materials.
Comparison with Alternatives
Other Piperidines
Chemists look at 3,5-dimethylpiperidine and compare it to other piperidines. Some examples are benzylpiperidine and 4-phenylpiperidine. These other piperidines often work better in lab tests. Benzylpiperidine can block enzymes more easily. It has lower IC50 values, so it is stronger. This helps a lot when making new medicines. Changing the piperidine ring or adding new groups can make these compounds work even better.
Scientists pick the best piperidine for each project. Sometimes, they want a stronger compound. Other times, 3,5-dimethylpiperidine is better because of its special shape. Its shape can help with selectivity or make it more stable.
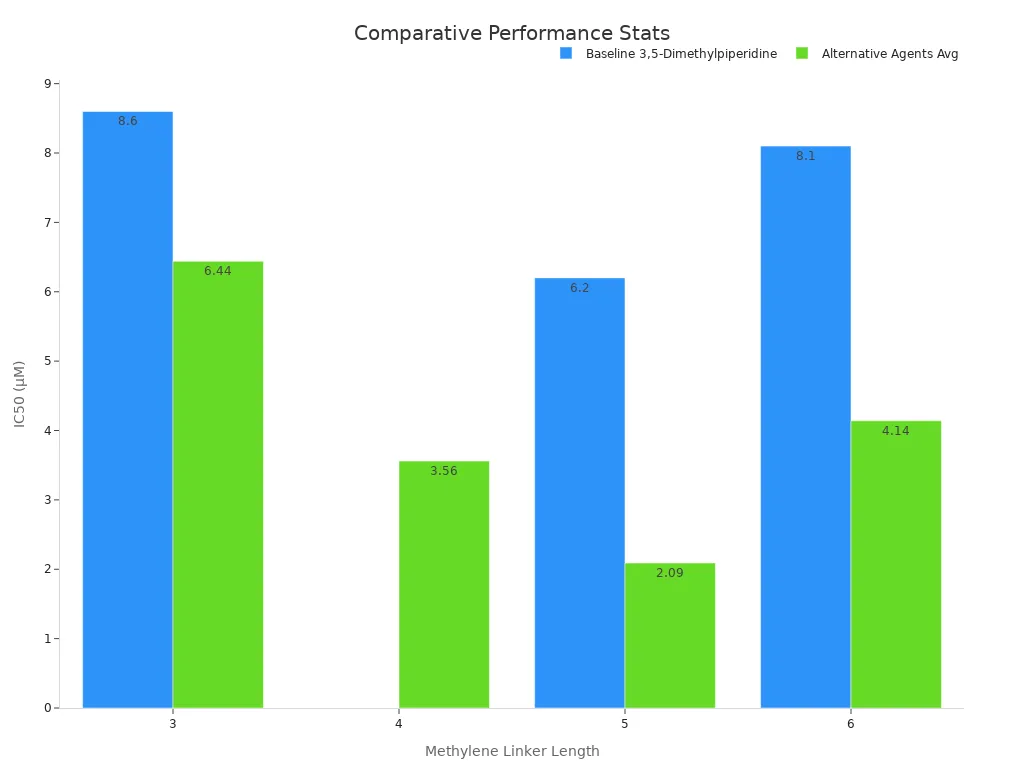
Non-piperidine Agents
Some agents are not piperidines, like N-ethylbenzylamine and N-(2-methoxybenzyl)ethanamine. These can also be used instead of 3,5-dimethylpiperidine. They are often even stronger. For example, N-ethylbenzylamine with a four-carbon linker has an IC50 of 0.92 μM. This is much lower than the 8.6 μM for 3,5-dimethylpiperidine. It means it can block enzymes using less material. Chemists use these agents when they need something very strong.
Summary Table
The table below shows how strong each compound is. Lower IC50 numbers mean the compound is stronger.
| Compound Type | Example Compound | IC50 (μM) | Relative Potency |
|---|---|---|---|
| Piperidine | Benzylpiperidine | 1.3–5.5 | More potent |
| Piperidine | 4-Phenylpiperidine | 3.6–6.4 | More potent |
| Non-piperidine | N-ethylbenzylamine | 0.81–4.3 | More potent |
| Non-piperidine | N-(2-methoxybenzyl)ethanamine | 0.62 | More potent |
| 3,5-Dimethylpiperidine | 3,5-Dimethylpiperidine | 6.2–8.6 | Baseline |
Chemists think about strength, selectivity, and other traits before picking a compound. 3,5-Dimethylpiperidine is still useful because of its special structure and many uses, even if some other compounds are stronger.
Practical Considerations
Handling and Safety
Chemists must be careful with 3,5-Dimethylpiperidine. Special packaging keeps it safe from water and air. Suppliers use containers that block air and moisture. They also use strong drums for storing the liquid. Warehouses keep it at cold or room temperatures to keep it stable. Safety papers, like MSDS and SDS, are in many languages. Labels follow world safety rules like REACH and GHS.
📦 Tip: Always read the label and safety sheet before you open a new container.
High purity (at least 99.0%) gives good lab results. Tests like HPLC and GC check the quality. Each batch is almost the same, with only small changes. TGA checks show it stays safe and lasts a long time.
Scalability
Factories make systems that can change for big or small orders. Two sites let them fill both small and large requests. You do not have to buy a lot at once. Rush orders help when you need it fast. Stackable IBCs make shipping big amounts easy.
- Flexible amounts help both labs and factories.
- Good planning stops delays and keeps supply steady.
- Free samples let people test before buying more.
Experts help with questions about use and handling. Each batch comes with papers like COA and DMF to follow the rules.
Cost and Availability
Suppliers show prices clearly and give discounts for big orders. Payment plans help buyers with their money. Special packaging and shipping save on costs. Most suppliers keep 3,5-Dimethylpiperidine ready to ship fast.
| Feature | Benefit |
|---|---|
| Volume discounts | Lower cost for larger orders |
| Flexible payment terms | Easier budget management |
| Custom packaging | Reduced shipping and storage expenses |
| Fast delivery | Reliable supply for urgent projects |
🛒 Note: You get help and rule papers with every order, so both small labs and big factories can use 3,5-Dimethylpiperidine easily.
3,5-Dimethylpiperidine is very important in organic synthesis today. Its special shape helps reactions work better and supports green chemistry. It also helps make new medicines and materials. The future for this compound looks good:
- New ways to make it give higher purity and better quality.
- More people want it because of new drug research and precision medicine.
- Green methods and tough rules make companies use safer production.
- The market could be worth $250 million by 2033, with Asia-Pacific, North America, and Europe growing the most.
Chemists think more people will use it as technology and industry change.
FAQ
What is the main use of 3,5-Dimethylpiperidine in organic synthesis?
Chemists use 3,5-Dimethylpiperidine in many ways. It helps make medicines and special chemicals. It also helps build advanced materials. Its structure lets it work as a building block, intermediate, and catalyst.
How do the cis and trans isomers affect its performance?
Cis and trans isomers have different shapes. Each shape can change how a reaction happens. The isomer picked can make a product stronger or weaker. Chemists choose the best isomer for each task.
Is 3,5-Dimethylpiperidine safe to handle in the lab?
Always use safety rules in the lab. Wear gloves and goggles to stay safe. Keep the compound in closed containers. Most labs say it is safe if you use it the right way.
Can factories produce 3,5-Dimethylpiperidine in large amounts?
Factories use new reactors and green ways to make big batches. Suppliers let you order small or large amounts. They also deliver quickly to labs and companies.