TMEDA, or N,N,N’,N’-Tetramethylethylenediamine, stands out in 2025 as a key player in modern chemistry. Chemists value TMEDA use for its strong coordination ability and low toxicity. In new solvent systems, TMEDA boosts CO2 absorption by about 10% and allows for higher amine concentrations, which helps lower costs. Its unique structure improves phase separation and energy efficiency during CO2 capture. These features make TMEDA a practical choice for labs and industry, where safety and performance matter most.
Key Takeaways
-
TMEDA is a versatile chemical that helps chemists make reactions faster, safer, and more selective by binding tightly to metals.
-
It improves organolithium chemistry by breaking up clusters, which increases reactivity and leads to higher yields in making complex molecules.
-
TMEDA supports important processes like polymerization, pharmaceutical synthesis, and metal complex formation, enhancing product quality and efficiency.
-
Its unique structure allows TMEDA to dissolve well in many solvents and stabilize reactive intermediates, making it a reliable tool in labs and industry.
-
Safe handling of TMEDA is essential; always follow safety guidelines like wearing gloves and goggles and checking the latest safety data sheets.
TMEDA Use Today
What is TMEDA?
TMEDA, or N,N,N’,N’-Tetramethylethylenediamine, is a small organic molecule that plays a big role in modern chemistry. Chemists recognize TMEDA as a colorless liquid with a faint amine odor. Its structure comes from ethylenediamine, but all four amine hydrogens are replaced by methyl groups. This change gives TMEDA unique properties. It acts as a bidentate ligand, which means it can attach to metal ions at two points. This ability allows TMEDA to form stable complexes with many metals, such as zinc chloride and copper(I) iodide.
TMEDA use has become essential in both research and industry. The molecule’s two nitrogen atoms, each with a lone pair of electrons, make it an excellent chelating agent. When TMEDA binds to a metal, it wraps around the metal ion, holding it tightly. This chelation stabilizes the metal and changes its reactivity. Chemists often choose TMEDA because it is easy to handle, has low toxicity, and dissolves well in many organic solvents.
Note: TMEDA is not just a laboratory curiosity. Its practical value extends to large-scale manufacturing and advanced research. Companies rely on TMEDA use to improve efficiency and safety in chemical processes.
TMEDA’s versatility comes from its structure. The methyl groups prevent the molecule from forming strong hydrogen bonds with itself or other molecules. This feature keeps TMEDA liquid at room temperature and helps it mix with a wide range of solvents. TMEDA’s ability to coordinate with metals, especially lithium and sodium, makes it a favorite in organometallic chemistry. It can break up clusters of organolithium compounds, making them more reactive and selective.
Chemists also use TMEDA as a base in many reactions. Its basicity is moderate, so it can accept protons without being too aggressive. This property allows TMEDA to control reaction rates and outcomes. In summary, TMEDA is a flexible, reliable tool for anyone working with metals or looking to fine-tune chemical reactions.
TMEDA at a Glance
| Property | Description |
|---|---|
| Chemical Formula | C6H16N2 |
| Structure | Two methylated amine groups linked by ethylene |
| Physical State | Colorless liquid |
| Key Functional Groups | Two tertiary amines |
| Main Role | Bidentate ligand, chelating agent, base |
| Solubility | Miscible with many organic solvents |
| Odor | Mild, amine-like |
Main Applications
TMEDA use spans a wide range of chemical processes. Its main applications focus on making reactions faster, more selective, and safer. Chemists and industry professionals turn to TMEDA for its ability to coordinate with metals, stabilize reactive intermediates, and control reaction pathways.
Organolithium Chemistry
One of the most important uses of TMEDA is in organolithium chemistry. Organolithium compounds are powerful reagents for making carbon-carbon bonds. However, these compounds often form large clusters that are less reactive. TMEDA breaks up these clusters by binding to the lithium ions. This process, called deaggregation, increases the reactivity and selectivity of the organolithium reagent. Chemists can then use these reagents to build complex molecules with greater precision.
Recent studies show that TMEDA use in organolithium chemistry leads to faster reactions and higher yields. For example, kinetic experiments reveal that TMEDA accelerates the elimination of cyclooctene oxide when sodium diisopropylamide (NaDA) is present. TMEDA’s chelating properties help stabilize the reactive intermediates, making the process more efficient. Mechanistic investigations confirm that TMEDA’s effect depends on how it binds to the reactants and transition states.
Phase-Transfer Catalysis and Metalation
TMEDA also plays a key role in phase-transfer catalysis. In these reactions, TMEDA helps move ions or molecules from one phase (like water) to another (like oil). This transfer allows reactions to happen that would otherwise be impossible or very slow. TMEDA’s ability to coordinate with metal ions makes it an effective phase-transfer catalyst, especially in reactions involving sodium or potassium.
In metalation reactions, TMEDA use helps control the formation of organometallic compounds. For example, TMEDA binds strongly to sodium diisopropylamide, forming a doubly chelated dimer. This complex is different from those formed with lithium analogs. Ligand exchange experiments show that TMEDA’s binding affinity to NaDA is about ten times stronger than that of dimethoxyethane (DME). This strong binding stabilizes the reagent and allows chemists to fine-tune the reactivity.
Polymerization and Catalysis
TMEDA use extends to polymerization reactions. In these processes, TMEDA acts as a modifier for catalysts. It improves the solubility of the catalyst and helps control the structure of the resulting polymer. For example, in olefin polymerization, TMEDA can influence the microstructure of the polymer, leading to better material properties.
Chemists also use TMEDA as a catalyst in organic polymerization. Its ability to coordinate with metals and stabilize reactive intermediates makes it valuable for producing high-quality polymers. TMEDA’s role as a base and ligand allows for precise control over the reaction conditions, which is important for making specialty polymers and materials.
Pharmaceutical Synthesis
TMEDA use is common in pharmaceutical synthesis. It enables regioselective metalation, which means chemists can add metals to specific positions on a molecule. This selectivity is crucial for making complex drug molecules. TMEDA’s ability to stabilize organosodium and organolithium reagents allows for the synthesis of intermediates that would be difficult to make otherwise.
Pharmaceutical companies rely on TMEDA to produce fine chemicals and active pharmaceutical ingredients (APIs). TMEDA acts as a coordination ligand and base, facilitating selective transformations. Its use in these processes helps ensure high purity and yield, which are essential for drug manufacturing.
Fine Chemical Production and Research
TMEDA use is not limited to large-scale manufacturing. It is also essential in research and development. Chemists use TMEDA to study new synthetic methods and explore the behavior of organometallic compounds. TMEDA’s stabilizing effect on transition metal complexes allows researchers to investigate new reactions and develop innovative materials.
In fine chemical production, TMEDA acts as a stabilizing ligand. It tunes the electronic properties and structural stability of metal complexes. This tuning is important for making specialty chemicals, catalysts, and advanced materials.
Dive Deeper: TMEDA’s Role in Modern Industry
TMEDA use in industry goes beyond the laboratory. Companies use TMEDA to improve the efficiency and safety of chemical manufacturing. For example, in the production of specialty polymers, TMEDA helps control the polymerization process. This control leads to materials with better performance and lower production costs.
Recent scientific studies highlight TMEDA’s practical value in industrial settings. Kinetic experiments show that TMEDA influences reaction rates, such as accelerating the elimination of cyclooctene oxide in the presence of NaDA. Mechanistic investigations reveal that TMEDA’s chelating properties contribute to catalytic effects. The extent of acceleration depends on the chelation state of the reactants and transition states.
Comparative studies with other polyamines, like PMDTA, show that TMEDA is especially effective in facilitating metalation and elimination reactions. Computed transition structures and isotopic labeling experiments support these findings. These results confirm that TMEDA use remains relevant in contemporary chemical manufacturing and research.
TMEDA in Numbers
| Application Area | Key Benefit | Recent Findings |
|---|---|---|
| Organolithium Chemistry | Enhanced reactivity and selectivity | Faster reactions, higher yields, better control |
| Phase-Transfer Catalysis | Efficient ion transfer | Improved reaction rates, broader applicability |
| Polymerization | Catalyst solubility, microstructure | Better material properties, lower costs |
| Pharmaceutical Synthesis | Regioselective metalation | Access to complex intermediates, high purity |
| Fine Chemical Production | Stabilizing ligand | Tuned electronic properties, structural stability |
TMEDA’s Benefits in Synthetic Chemistry
TMEDA use brings several benefits to synthetic chemistry:
-
Stabilizes Reactive Intermediates: TMEDA forms stable complexes with metals, preventing unwanted side reactions.
-
Controls Aggregation: By breaking up clusters of organolithium or organosodium compounds, TMEDA increases reactivity.
-
Enables Tunable Reactivity: Chemists can adjust reaction rates and selectivity by changing the amount of TMEDA.
-
Improves Solubility: TMEDA helps dissolve metal reagents in organic solvents, making reactions more efficient.
-
Facilitates Ligand Exchange: TMEDA can be replaced by other ligands, allowing for controlled changes in reactivity.
These benefits are supported by NMR spectroscopy, titration methods, and density functional theory (DFT) computations. For example, titration studies show a linear relationship between TMEDA concentration and NaDA dissolution. Molecular weight measurements confirm the formation of doubly chelated dimers. DFT calculations reveal that ligand displacement by ethereal ligands, such as tetrahydrofuran (THF), is exothermic and accelerates metalation rates.
TMEDA vs. Other Ligands
Chemists often compare TMEDA use with other ligands, such as dimethoxyethane (DME) and pentamethyldiethylenetriamine (PMDTA). TMEDA’s binding affinity to NaDA is about ten times stronger than DME. This strong binding stabilizes the reagent but can limit solubility at low temperatures. TMEDA’s ligand substitution lability means it can be displaced by other ligands, allowing for fine control over reaction conditions.
| Ligand | Binding Affinity to NaDA | Solubility at Low Temp | Ligand Exchange Rate |
|---|---|---|---|
| TMEDA | High | Limited | Moderate |
| DME | Low | Good | Fast |
| PMDTA | Moderate | Moderate | Slow |
Chemists choose TMEDA when they need strong binding and controlled reactivity. They may switch to DME or PMDTA for different solubility or exchange properties.
TMEDA in the Future
TMEDA use continues to grow as new applications emerge. Researchers are exploring TMEDA’s role in advanced materials, such as metal-organic frameworks and nanomaterials. Its ability to stabilize metal complexes and control reactivity makes it a valuable tool for developing new technologies.
Chemical Properties
Structure and Chelation
TMEDA has a simple but powerful structure. It contains two nitrogen atoms, each bonded to two methyl groups. These nitrogen atoms sit at each end of an ethylene bridge. This setup lets TMEDA act as a bidentate ligand, which means it can grab onto a metal ion at two points. Chemists often see TMEDA form stable rings with metals like lithium, sodium, copper, and silver.
Recent NMR spectroscopy studies have shown that TMEDA forms cyclic dimers with lithium enolates, phenolates, and alkoxides. These experiments used low-temperature 6Li NMR to reveal how TMEDA wraps around lithium ions. The results help chemists understand why TMEDA stabilizes these reactive species so well. Single crystal X-ray studies also confirm that TMEDA binds to copper(I) and silver(I) through both nitrogen atoms. This chelation creates a four-coordinate metal center, which is very stable.
Other molecular data, like 1H and 19F NMR, show that TMEDA can form tetrameric complexes with sodium enolates. Similar molecules without this chelating ability cannot make these stable structures. Positron Annihilation Lifetime (PAL) spectroscopy even shows that TMEDA’s chelation changes the electronic structure of its complexes. This evidence proves that TMEDA’s structure is key to its strong chelating power.
Tip: Chemists choose TMEDA when they need a ligand that can hold metals tightly and create stable, well-defined complexes.
Solubility and Reactivity
TMEDA dissolves in many organic solvents. Its two tertiary amine groups help it mix with both polar and nonpolar liquids. However, TMEDA does not dissolve well in water because it lacks hydroxyl groups. The methyl groups on the nitrogen atoms make it less likely to form hydrogen bonds with water.
Chemists have measured TMEDA’s solubility using vapor-liquid equilibrium (VLE) experiments. These studies show that temperature, CO2 pressure, and amine concentration all affect how much TMEDA dissolves. Titration curves reveal that TMEDA can accept one or two protons, which confirms its acid-base properties. NMR spectroscopy helps chemists track how TMEDA changes in solution, especially when it reacts with CO2.
-
CO2 absorption experiments show that TMEDA reacts with CO2 in water.
-
NMR studies reveal how TMEDA’s structure changes during these reactions.
-
Titration data confirm TMEDA’s ability to accept protons.
-
VLE measurements give exact solubility values at different temperatures and pressures.
-
Thermodynamic analysis provides details about TMEDA’s protonation and dissociation.
Comparative studies with other amines show that TMEDA’s structure gives it unique solubility and reactivity. It absorbs CO2 better than some monoamines, but not as well as diamines with hydroxyl groups. Chemists use this information to pick the right amine for each job.
| Property | TMEDA Value | Notes |
|---|---|---|
| Water Solubility | Low | Lacks hydroxyl groups |
| Organic Solvent Solubility | High | Mixes with many solvents |
| CO2 Absorption | Moderate | Outperforms some monoamines |
| Protonation States | Mono- and diprotonated | Confirmed by titration and NMR |
| Reactivity with Metals | Strong chelation | Forms stable complexes |
TMEDA’s chemical properties make it a favorite for chemists who need a reliable, versatile ligand that works in many environments.
TMEDA in Synthesis
Organolithium Chemistry
Organolithium chemistry stands as one of the most important areas where TMEDA use has transformed synthetic strategies. Chemists often face challenges when working with organolithium reagents. These compounds tend to form large aggregates or clusters, which can reduce their reactivity and make reactions less predictable. TMEDA steps in as a chelating agent and ligand, breaking up these clusters and creating more reactive species.
When TMEDA binds to lithium ions, it forms stable, well-defined complexes. This chelation process increases the solubility of organolithium reagents in organic solvents. As a result, reactions proceed faster and with greater selectivity. Chemists can control the outcome of their reactions by adjusting the amount of TMEDA added. For example, using a higher ratio of TMEDA to lithium can lead to more complete deaggregation, which means more reactive lithium species are available for the reaction.
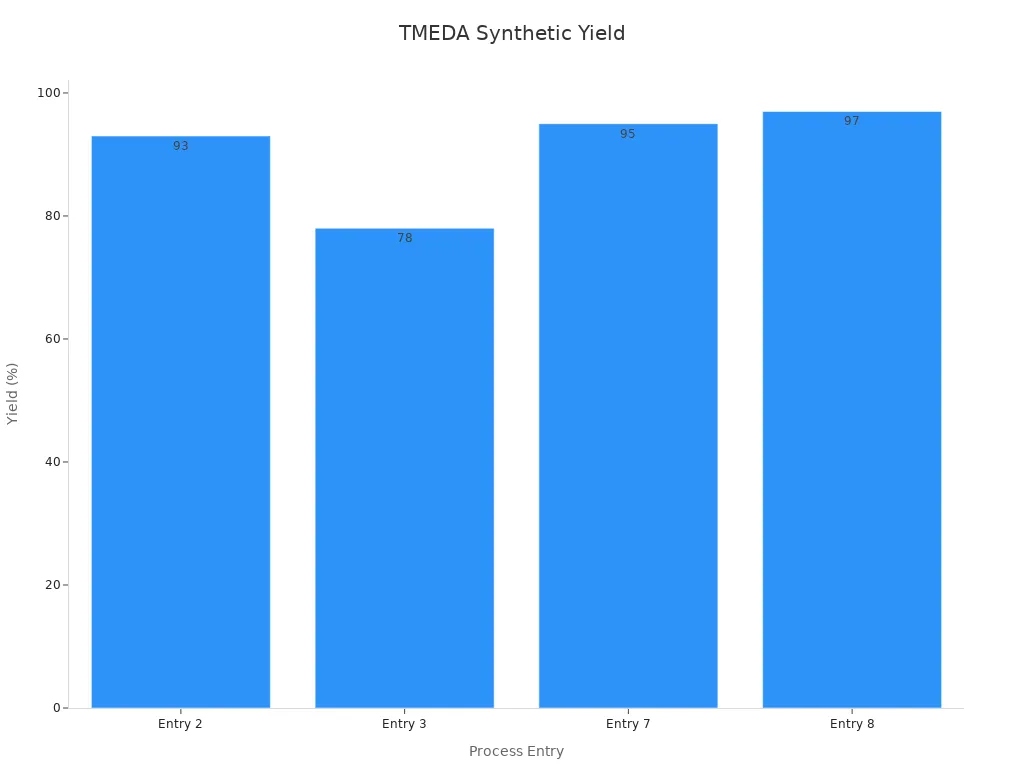
Let’s look at a real-world example. In the direct synthesis of ortho-methylene-bridged N-heterobiaryls from N-heterocycles, adding TMEDA as a chelating agent dramatically boosts the yield. Without any chelating agent, the yield sits at a disappointing 7%. When chemists add TMEDA (1.3 equivalents) in toluene at 120°C for 2 hours, the yield jumps to 93%. Even in hexane, the yield climbs to 78%. Other chelating agents like DABCO or PMDTA do not come close to TMEDA’s performance.
| Entry | Chelating Agent (equiv) | Solvent | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | None | Hexane | 120 | 2 | 7 |
| 2 | TMEDA (1.3) | Toluene | 120 | 2 | 93 |
| 3 | TMEDA (1.3) | Hexane | 120 | 2 | 78 |
| 4 | DABCO (1.3) | Hexane | 120 | 2 | 7 |
| 5 | PMDTA (1.3) | Hexane | 120 | 2 | 15 |
| 6 | DMEDA (1.3) | Hexane | 120 | 2 | 0 |
| 7 | TMEDA (0.2) | Toluene | 140 | 14 | 95 |
| 8 | TMEDA (0.1) | Toluene | 140 | 14 | 97 |
This data tells a clear story. TMEDA use in organolithium chemistry does not just improve yields; it changes the entire reaction landscape. Chemists can now access products that were once difficult or impossible to make. The ability to fine-tune reactivity and selectivity gives researchers more control over complex syntheses.
Tip: When working with sensitive organolithium reagents, adding TMEDA can make the difference between a failed experiment and a high-yielding, clean reaction.
Chemists also appreciate TMEDA’s moderate basicity. It acts as a base without being too aggressive, which helps prevent unwanted side reactions. This property becomes especially important in multi-step syntheses, where selectivity matters. TMEDA’s role as both a ligand and a base makes it a versatile tool in the synthetic chemist’s toolkit.
Dive Deeper: TMEDA’s Impact on Modern Organolithium Synthesis
In recent years, researchers have pushed the boundaries of what organolithium chemistry can achieve. TMEDA use has played a central role in these advances. For example, in the synthesis of complex pharmaceutical intermediates, TMEDA helps stabilize reactive lithium species long enough for them to react with delicate substrates. This stabilization leads to higher yields and fewer byproducts.
Chemists have also explored the use of TMEDA in asymmetric synthesis. By pairing TMEDA with chiral ligands, they can create organolithium reagents that favor the formation of one enantiomer over another. This selectivity is crucial for making drugs and fine chemicals with the desired biological activity.
TMEDA’s influence extends beyond the laboratory. In industrial settings, companies rely on TMEDA to streamline large-scale organolithium reactions. The improved solubility and reactivity reduce the need for excess reagents, which cuts costs and minimizes waste. Safety also improves because TMEDA helps control the reactivity of potentially hazardous organolithium compounds.
Researchers continue to study the fundamental chemistry of TMEDA-lithium complexes. Advanced techniques like NMR spectroscopy and X-ray crystallography reveal the structures of these complexes in detail. These studies help chemists design new reactions and predict how TMEDA will behave in different environments.
Coordination and Advanced Materials
Metal Complexes
TMEDA shows strong performance as a ligand in metal complex formation. Chemists often use TMEDA to stabilize metals like iron, copper, and tantalum. This stability helps create new catalysts and materials. Recent research highlights TMEDA’s unique role in iron catalysis. Scientists used low-temperature synthesis and advanced spectroscopy to observe TMEDA-ligated iron species. These species drive selective C(sp2)−C(sp3) cross-coupling reactions. The study used 57Fe Mössbauer spectroscopy and X-ray diffraction to confirm TMEDA’s direct involvement. TMEDA forms and stabilizes the active iron complexes, making the reaction possible.
Another study focused on hydromagnesiation reactions. TMEDA enabled the formation of iron(II)-alkyl species. These species then reduced to iron(0) complexes, which act as the true catalysts. Without TMEDA, these active forms did not appear. This shows TMEDA’s essential function in creating and maintaining effective metal catalysts.
Chemists also validate TMEDA’s coordination with metals using quantitative methods. For example, the TaCl4(TMEDA) complex was isolated in high yield. 1H NMR spectroscopy showed clear chemical shifts, and X-ray crystallography confirmed the structure. UV-vis and EPR spectroscopies provided more details about the electronic properties. Hirshfeld surface analysis helped quantify the interactions in these complexes.
| Metal Complex | Method of Validation | Key Finding |
|---|---|---|
| Fe-TMEDA | Mössbauer, XRD, GC | Forms/stabilizes active catalyst |
| TaCl4(TMEDA) | NMR, XRD, UV-vis, EPR | High yield, confirmed structure |
TMEDA’s ability to coordinate with metals gives chemists a reliable tool for building stable and active complexes.
Advanced Material Applications
TMEDA’s coordination chemistry supports the development of advanced materials. Chemists use TMEDA-ligated complexes to create new reactions with high regio- and stereoselectivity. These features are important for designing materials with precise structures and functions. TMEDA’s role in organometallic complexes helps produce specialty polymers, electronic materials, and catalysts.
Industry trends show growing demand for TMEDA in high-performance polymers and electronics. The South Korean market report points to TMEDA’s critical role in pharmaceuticals, specialty chemicals, and semiconductors. Companies value TMEDA for its ability to improve catalytic processes and support sustainable material development.
Researchers also explore TMEDA’s impact on bio-based materials. In one experiment, adding 1 mM TMEDA to a CO2 absorption-microalgae system increased microalgal biomass by nearly 20%. Carbon conversion efficiency rose by about 49%. This result suggests TMEDA can boost bio-material production and help create greener technologies.
| Application Area | TMEDA’s Benefit | Recent Result |
|---|---|---|
| Polymers/Electronics | High selectivity, stability | Increased demand, better performance |
| Bio-based Materials | Enhanced CO2 absorption, growth | +20% biomass, +49% efficiency |
TMEDA’s versatility makes it a key ingredient in the next generation of advanced and sustainable materials.
Safety and Handling
Safe Practices
TMEDA plays a big role in many labs and factories, so safe handling matters. Workers often follow strict guidelines to keep everyone safe. Material Safety Data Sheets (MSDS) give clear instructions for handling TMEDA. These sheets list the hazards and show how to control them. People who work with TMEDA use special gear to protect themselves.
-
Gloves keep TMEDA off the skin.
-
Safety goggles protect the eyes from splashes.
-
Good ventilation helps remove fumes from the air.
-
Respirators may be needed if the air has too much TMEDA.
-
Workers wash hands after handling TMEDA and before eating.
Companies also set up chemical hygiene plans. These plans require regular reviews of MSDS, usually every three years. This keeps the information up to date. Training sessions teach workers how to handle TMEDA safely. Everyone learns what to do if there is a spill or accident.
Tip: Always check the MSDS before starting work with TMEDA. This habit helps prevent mistakes and keeps everyone safe.
Regulatory Updates
Rules for handling TMEDA keep changing as new research comes out. In 2025, many countries follow OSHA standards for chemical safety. These rules make sure that manufacturers provide detailed MSDS for TMEDA. The sheets must list all hazard components and set threshold limit values (TLVs). TLVs show how much TMEDA can be in the air before it becomes unsafe.
| Requirement | Description |
|---|---|
| MSDS | Lists hazards, controls, and safe practices |
| TLVs | Sets safe exposure limits for workers |
| OSHA Compliance | Enforces regular MSDS updates and training |
Regulators check that companies follow these rules. If a company does not meet the standards, it must fix the problem right away. These steps help protect workers and the environment. TMEDA stays a safe choice when everyone follows the latest safety and regulatory guidelines.
TMEDA use continues to shape modern chemistry in 2025. Chemists and industry leaders rely on it for better yields, safer processes, and new materials. They see TMEDA as a trusted tool for both research and manufacturing. Looking ahead, experts expect more innovation in green chemistry and advanced materials. Safety awareness will stay important as TMEDA finds new roles in labs and factories.
FAQ
What makes TMEDA different from other amine ligands?
TMEDA stands out because it has two methylated amine groups. These groups help TMEDA grab metal ions tightly. This strong grip makes TMEDA a favorite for chemists who want stable and reactive metal complexes.
Can TMEDA be used in water-based reactions?
TMEDA does not dissolve well in water. Chemists usually use it in organic solvents. If someone needs a water-soluble ligand, they often pick a different amine.
Is TMEDA safe to handle in the lab?
TMEDA is safer than many other chemicals, but it still needs careful handling.
Always wear gloves and goggles. Work in a well-ventilated area. Check the MSDS for the latest safety tips.
Where do chemists use TMEDA most often?
Chemists use TMEDA in many places. Here are some common uses:
-
Organolithium chemistry
-
Metal complex formation
-
Polymerization reactions
-
Pharmaceutical synthesis
| Application Area | Why Use TMEDA? |
|---|---|
| Organolithium Reactions | Boosts reactivity |
| Polymerization | Improves control |
| Metal Complexes | Stabilizes metals |
| Pharmaceuticals | Enables selectivity |