Anyone who’s spent time in a synthesis lab knows the frustration of reduction reactions gone wrong. Selective reduction remains one of the trickier challenges in fine chemical synthesis, with conventional reagents often proving too aggressive or unpredictable.
DIBAL-H (Diisobutylaluminum Hydride)[^1] has become the go-to solution for chemists needing to convert esters or nitriles to aldehydes without destroying nearby functional groups.
I’ve seen countless synthesis projects rescued by switching to DIBAL-H. It’s not just another reagent—it’s the difference between a failed batch and a successful production run.
What Is DIBAL-H and Why Is It Important?
I remember a pharmaceutical client who had tried everything to reduce their complex ester intermediate[^2]. Their yields were terrible until they discovered the selective power of DIBAL-H.
DIBAL-H, or Diisobutylaluminum Hydride, lets chemists perform what once seemed impossible: the clean conversion of esters or nitriles to aldehydes without overreduction.
DIBAL-H (CAS 1191-15-7) isn’t new—it’s been around for decades—but its importance in modern synthesis continues to grow. Unlike the sledgehammer approach of LiAlH₄ or the unpredictability of NaBH₄ in complex molecules, DIBAL-H offers surgical precision when you need to stop at the aldehyde oxidation state.
Basic Chemical Information
| Property | Value |
|---|---|
| Chemical Name | Diisobutylaluminum Hydride |
| CAS Number | 1191-15-7 |
| Molecular Formula | C8H19Al |
| Appearance | Clear to slightly yellow liquid |
| Storage Conditions | Inert atmosphere, ≤25°C |
Chemical Structure
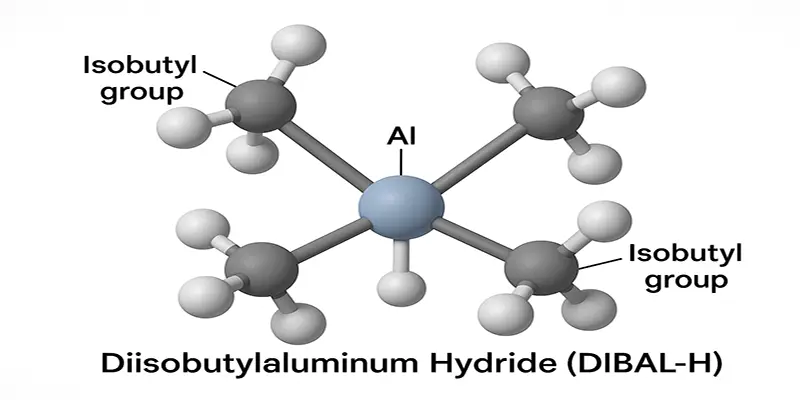
The magic of DIBAL-H lies in its structure. Those two bulky isobutyl groups flanking the aluminum center aren’t just there by accident—they create the steric hindrance that prevents the reagent from delivering its second hydride too easily. This structural feature is what gives DIBAL-H its characteristic selectivity.
Selectivity Mechanism
The temperature sensitivity of DIBAL-H reactions isn’t just a precaution—it’s essential to its function. At -78°C (dry ice/acetone bath temperature), the reaction kinetics slow down enough that you can catch the reaction at the tetrahedral intermediate stage. Let it warm up too quickly, and you’ll end up with alcohols instead of the desired aldehydes.
We’ve found that most customers prefer the hexane solution for general applications, though toluene formulations work better for reactions requiring higher temperatures.
How Does DIBAL-H Compare to Other Reducing Agents?
Last month, one of our customers switched from LiAlH₄ to DIBAL-H for a key step in their API synthesis. The result? Their yield jumped from 63% to 91%, and they eliminated a troublesome purification step.
DIBAL-H fills the gap between overly aggressive reducers like LiAlH₄ and weaker options like NaBH₄, offering the perfect balance for aldehyde synthesis.
Here’s how DIBAL-H stacks up against the alternatives:
Comparative Table of Reducing Agents
| Reagent | Selectivity | Typical Use | Hazards | Temperature Range |
|---|---|---|---|---|
| DIBAL-H | High | Ester/Nitrile → Aldehyde | Air/water sensitive | -78°C to 0°C |
| LiAlH₄ | Low | Broad-spectrum reduction | Pyrophoric | 0°C to RT |
| Red-Al | Medium | Complex reductions | Moisture sensitive | -20°C to RT |
| NaBH₄ | Medium-Low | Ketone/Aldehyde → Alcohol | Low water sensitivity | 0°C to RT |
Why Selectivity Matters
In the real world of pharmaceutical synthesis, selectivity isn’t just about chemical elegance—it’s about economics. When you’re working with intermediates that might cost thousands per kilogram, the ability to stop at the aldehyde stage rather than reducing all the way to an alcohol can make or break your process economics.
A process chemist at one of our European clients told me they had tried three different reducing agents before DIBAL-H. "Nothing else gave us the clean NMR profile we needed," she explained. "With DIBAL-H, we finally got consistent results batch after batch."
What Are the Main Applications of DIBAL-H?
The versatility of DIBAL-H continues to surprise me. Just when I think I’ve seen all its applications, a customer comes along with an innovative new use.
From steroid side chains to polymer end-group modifications, DIBAL-H has become the selective reducer of choice across pharmaceutical, agrochemical, and specialty materials industries.
DIBAL-H shines in both R&D labs and commercial manufacturing settings:
Key Application Areas
| Industry | Common Use Case | Real-World Example |
|---|---|---|
| Pharmaceuticals | Macrolide, steroid, and antiviral intermediate production | Used in the synthesis of oseltamivir (Tamiflu) intermediates |
| Agrochemicals | Preparation of aromatic aldehydes | Key step in pyrethroids production |
| Materials Chemistry | Modification of polymer end groups | Creating reactive handles on PEG chains |
| Flavors & Fragrances | Creation of aldehyde-containing compounds | Synthesis of vanillin derivatives |
Reaction Example
The conversion of methyl benzoate to benzaldehyde looks simple on paper:
Methyl benzoate + DIBAL-H → Benzaldehyde
But anyone who’s actually run this reaction knows the devil is in the details. Temperature control is critical—I’ve seen labs struggle for weeks with inconsistent results until they invested in proper cooling equipment. Keep it below -40°C during addition, and you’ll typically see yields above 90%.
We offer both research-grade (>98% purity) and industrial-grade (>95% purity) formulations to match different application needs.
How Should DIBAL-H Be Handled and Stored?
I’ll never forget visiting a lab where they’d stored DIBAL-H improperly. The resulting cleanup and safety investigation shut down their operations for a week.
DIBAL-H demands respect—proper storage under nitrogen or argon at controlled temperatures isn’t just recommended, it’s essential for safety and reagent quality.
DIBAL-H reacts vigorously with water, releasing hydrogen gas and generating heat. This isn’t just chemistry trivia—it’s a serious safety consideration that demands proper handling techniques.
Packaging and Transportation
- Standard packaging: 25kg drum, 100kg drum
- Carrier solvents: Toluene or hexane
- Hazard classification: UN 2924, Class 3 (flammable liquid)
- Labeling: GHS02 (Flammable), GHS05 (Corrosive)
| Parameter | Value | Practical Implication |
|---|---|---|
| UN Number | 2924 | Requires hazardous materials shipping documentation |
| Hazard Class | 3 (Flammable Liquid) | Must be stored away from ignition sources |
| Packing Group | II | Medium danger level for transportation |
| Shipping Method | Sea or Air (with hazardous goods declaration) | Air shipment requires special arrangements |
I’ve seen customers struggle with import delays because they weren’t prepared for the documentation requirements. Our logistics team specializes in hazardous chemical shipments and can guide you through the process to avoid customs holdups.
Can DIBAL-H Be Customized for Industrial Use?
A Japanese customer recently approached us with an unusual request: they needed DIBAL-H in cyclopentyl methyl ether for a specialized continuous flow process. We hadn’t done it before, but we made it happen.
Yes, DIBAL-H formulations can be tailored to specific process needs—from solvent selection to concentration adjustments that match your exact reaction conditions.
No two manufacturing processes are identical. We’ve worked with clients across four continents to develop customized DIBAL-H formulations that fit their specific equipment and processes:
Options We Offer
- Solvent: Beyond standard hexane or toluene, we’ve worked with cyclopentyl methyl ether and 2-methyltetrahydrofuran
- Concentration: While 1.0M is standard, we’ve produced concentrations from 0.5M for sensitive applications to 2.0M for space-limited facilities
- Stabilization: Some processes benefit from trace additives that extend shelf life
- Drum Type: From standard 25L drums to custom 1000L IBCs for large-scale operations
One pharmaceutical manufacturer in India told me they saved nearly 30% on their process costs by switching to our custom 1.8M formulation, which reduced solvent handling and waste treatment requirements.
What Regulatory and Documentation Support Is Available?
Regulatory hurdles can be as challenging as chemical ones. I recently helped a customer navigate a complex REACH inquiry that had stalled their production for weeks.
Beyond the molecule itself, we provide the comprehensive documentation package needed to satisfy regulators and quality departments worldwide.
Every shipment includes documentation that meets or exceeds international standards:
Standard Documents
- Certificate of Analysis (COA) with batch-specific analytical results
- Safety Data Sheet (MSDS) in your local language and format
- Technical Data Sheet (TDS) with application guidance
- Certificate of Origin (COO) for customs clearance
- REACH pre-registration confirmation for European customers
- Packing Declaration with container certification details
We also provide:
- Free samples (typically 100-250g) for process validation
- CIQ certification for shipments to China
- Custom specifications testing when standard analytics aren’t sufficient
A quality manager at a Swiss pharmaceutical company mentioned that our documentation was the most complete they’d received from any chemical supplier—which made their audit preparation significantly easier.
Why Do Clients Choose TUODA as Their DIBAL-H Supplier?
After 15 years in this business, I’ve learned that chemistry is just part of the equation. Reliability and service often matter just as much as molecular structure.
TUODA has earned its reputation through consistent quality, responsive service, and the flexibility to solve problems that other suppliers consider "too difficult."
Our customers consistently highlight several reasons they choose us:
- Technical partnership: Our team includes chemists who have actually used DIBAL-H in manufacturing settings—we understand your challenges firsthand
- Supply security: With production facilities in multiple locations, we’ve maintained 100% delivery reliability even during recent global supply chain disruptions
- Quality consistency: Our proprietary purification process ensures batch-to-batch consistency with metal impurities below 10ppm
- Responsive service: When a customer in Germany had an unexpected production opportunity, we airshipped material within 48 hours of their call
"What impressed me wasn’t just the quality of the product," said the procurement director at a major US pharmaceutical company, "but how quickly they responded when we had questions about an unusual application. Their technical team actually helped us optimize our process."
Conclusion
DIBAL-H represents that rare combination in chemistry—powerful enough to be broadly useful, yet selective enough to be precisely controlled. Whether you’re developing a new API or scaling up an established process, the right reducing agent can make all the difference between success and frustration.
If you’re working with reductions and haven’t explored what DIBAL-H can offer your specific application, you might be missing an opportunity to improve both your chemistry and your process economics. Our technical team is always available to discuss your particular challenges.
[^1]: Explore this resource to understand the significance of DIBAL-H in selective reduction and its applications in various industries.
[^2]: Learn about the reduction of ester intermediates and the role of DIBAL-H in improving yields and selectivity in synthesis processes.